Thermo 3 teil 2
Mass flow (velocity triangle)
m˙ = ρ · A · C_x
Ideal pump work
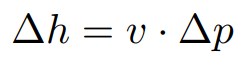
Isentropic ideal gas equations T_2/T_1 to v
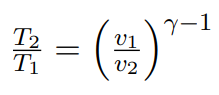
Isentr. turbine efficiency
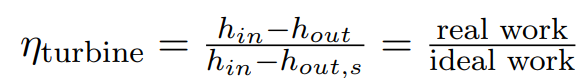
Specific Enthalpy of Gases
h = u + pv
Isentropic Compression
h2 = h1 + v1 (p2 − p1)
Thermal efficiency
ηthermal =W_out/Q_in
Nassdampfgebiet mit x interpolieren:
h = h_f + x(h_g-h_f)
Ideal Gas equations
pv = RT
p = ρRT
Isentr. compressor efficiency
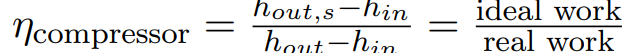
1st Law of Thermodynamics
W˙ − Q˙ = ˙m ( h1 − h2 + (v12−v22)/ρ + g (z1 − z2) )
Thermal coefficients
R = cp − cv
γ = k = cp/cv
Euler work
delta_h = U*delta C_theta
Linear interpolation
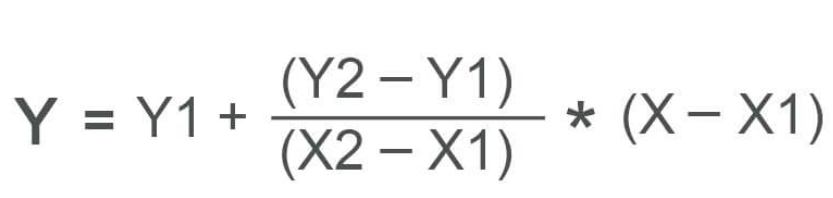
Isentropic ideal gas equations T_2/T_1 to p
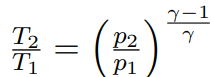
Isentropic ideal gas equations p_2/p_1 to v
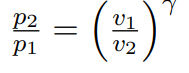
Nassdampfanteil X
X = (s-s_f)/)s_g-s_f)
Rotational speed
U = ω · r = 2π · f · r
const volume / pressure
h = c*T